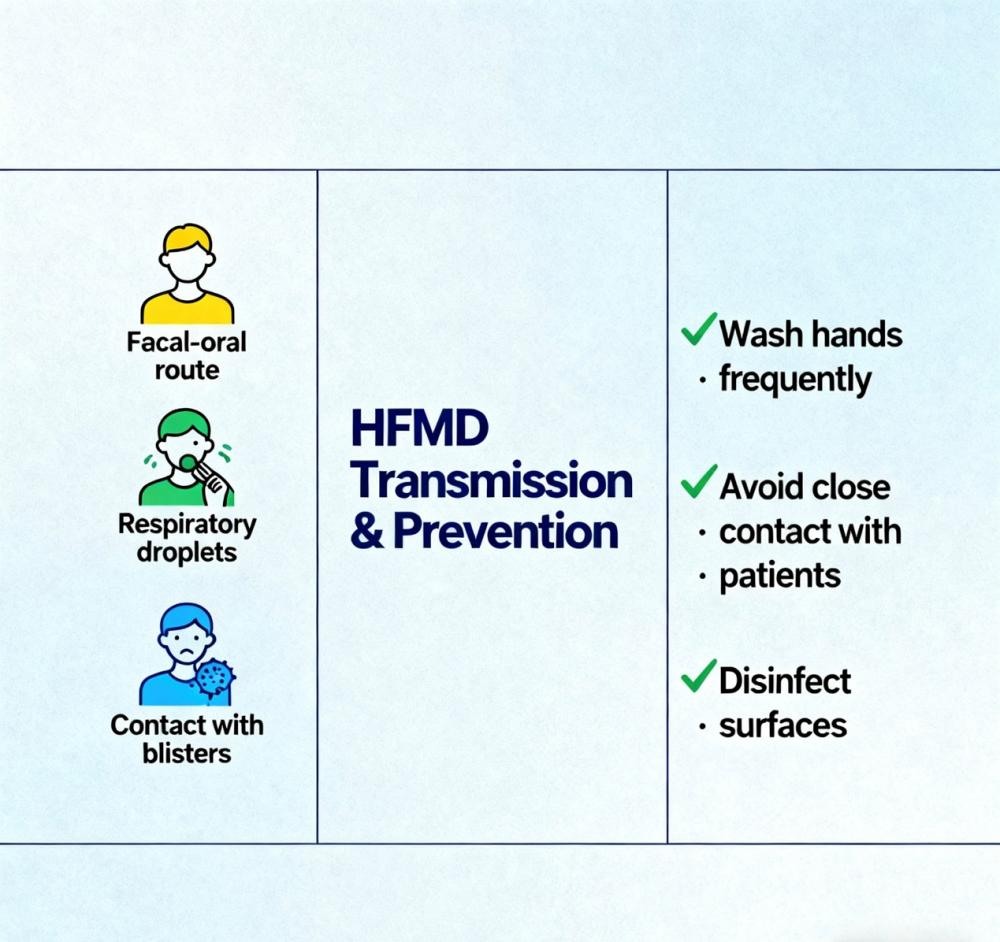
Hand, Foot, and Mouth Disease (HFMD) Overview
Hand, Foot, and Mouth Disease is primarily prevalent among young children. It is highly contagious, has a large proportion of asymptomatic infections, complex transmission routes, and rapid spread, potentially causing widespread outbreaks within a short period, making epidemic control challenging. During outbreaks, collective infections in kindergartens and childcare centers, as well as familial clustering of cases, can occur. In 2008, HFMD was included by the Ministry of Health in the management of Category C infectious diseases.
Coxsackievirus A16 (CA16) and Enterovirus 71 (EV71) are common viruses causing HFMD. Epidemiological data indicate that CA16 often circulates concurrently with EV71, leading to frequent HFMD outbreaks. During these outbreaks, the proportion of CA16 infections far exceeds that of EV71, often accounting for over 60% of total infections. HFMD caused by EV71 can lead to central nervous system damage. The proportion of severe cases and the case fatality rate among patients infected with EV71 are significantly higher than those infected with other enteroviruses, with severe case fatality rates reaching 10%-25%. However, CA16 infection generally does not cause various central nervous system-related diseases such as aseptic meningitis, brainstem encephalitis, and poliomyelitis-like paralysis. Therefore, early differential diagnosis is particularly crucial for saving the lives of severe cases.
Clinical Testing
Current clinical testing for HFMD primarily involves nucleic acid detection of the pathogen and serological antibody detection. Beier company utilizes enzyme-linked immunosorbent assay (ELISA) and colloidal gold methods to develop Enterovirus 71 Antibody Test Kits and Coxsackievirus A16 IgM Antibody Test Kits for the differential detection of HFMD pathogens. Serum antibody detection offers high sensitivity, good specificity, and is simple, rapid, and suitable for clinical testing in healthcare institutions at all levels and for large-scale epidemiological surveillance studies.
Specific Diagnostic Indicators and Clinical Significance of EV71 Infection
The specific diagnosis of EV71 infection relies on the detection of EV71-RNA, EV71-IgM, and EV71-IgG antibodies in serum, or the detection of EV71-RNA in swab specimens.
Following EV71 infection, IgM antibodies appear first, peaking in the second week. IgG antibodies begin to appear in the second week post-infection and persist for a relatively long time. EV71-IgM is an important indicator of primary or recent infection, facilitating early detection and treatment of EV71 infection. EV71-IgG is a key indicator for the differential diagnosis of infection, useful for epidemiological investigation and evaluation of vaccination efficacy. Detecting the change in antibody titer between paired acute and convalescent serum samples can also determine EV71 infection status; for instance, a four-fold or greater geometric increase in antibody titer in convalescent serum compared to acute serum can be judged as a current EV71 infection.
Specific Diagnostic Indicators and Clinical Significance of CA16 Infection
The specific diagnosis of CA16 infection relies on the detection of CA16-RNA, CA16-IgM, and CA16-IgG antibodies in serum, or the detection of CA16-RNA in swab specimens.
Following CA16 infection, IgM antibodies appear first, peaking in the second week. IgG antibodies begin to appear in the second week post-infection and persist for a relatively long time. CA16-IgM is an important indicator of primary or recent infection.
Significance of Combined EV71 and CA16 Antibody Testing
HFMD is caused by several enteroviruses, with common serotypes being EV71 and CA16. Research indicates that HFMD caused by CA16 virus typically presents with relatively classic symptoms, has fewer complications, and a good prognosis. In contrast, HFMD caused by EV71 often presents with more severe clinical symptoms, has a higher rate of severe cases and case fatality, and is frequently associated with central nervous system complications. The clinical symptoms of HFMD are complex and often lack typicality, making clinical diagnosis particularly challenging, especially in the early stages. The significance of combined serum antibody testing lies in replacing the time-consuming and cumbersome traditional virus isolation methods, identifying the pathogen serologically, and providing a basis for clinical diagnosis, treatment strategies, and disease prognosis.
Product Performance Analysis
EV71-IgM ELISA Kit Performance Analysis
|
Sample |
No. of Cases |
EV71-IgM Positive |
EV71-IgM Negative |
Sensitivity |
Specificity |
|
Confirmed EV71 Cases |
302 |
298 |
4 |
98.7% |
—– |
|
Non-EV71 Infection Cases |
25 |
1 |
24 |
—– |
96% |
|
General Population |
700 |
—– |
700 |
—– |
100% |
Results indicate: The Beier EV71-IgM Test Kit demonstrates high sensitivity and good specificity for testing serum from EV71-infected individuals. Data source: National Institute for Viral Disease Control and Prevention, Chinese CDC.
EV71-IgG ELISA Kit Performance Analysis (I)
|
Sample |
No. of Cases |
EV71-IgG Positive |
EV71-IgG Negative |
Sensitivity |
Specificity |
|
Confirmed EV71 Cases |
310 |
307 |
3 |
99.0% |
—– |
|
Non-EV71 Infection Cases |
38 |
0 |
38 |
—– |
100% |
|
General Population |
700 |
328 |
372 |
—– |
100% |
EV71-IgG ELISA Kit Performance Analysis (II)
|
Sample |
No. of Cases |
EV71-IgG Positive |
EV71-IgG Negative |
Sensitivity |
Specificity |
|
General Population, Neutralization Test Positive |
332 |
328 |
4 |
98.8% |
—– |
|
General Population, Neutralization Test Negative |
368 |
—– |
368 |
—– |
100% |
Results indicate: The Beier EV71-IgG Test Kit demonstrates a high detection rate for serum from individuals with recurrent EV71 infection. Data source: National Institute for Viral Disease Control and Prevention, Chinese CDC.
CA16-IgM ELISA Kit Performance Analysis
|
Sample |
No. of Cases |
CA16-IgM Positive |
CA16-IgM Negative |
Sensitivity |
Specificity |
|
Confirmed CA16 Cases |
350 |
336 |
14 |
96.0% |
—– |
|
General Population |
659 |
0 |
659 |
—– |
100% |
Results indicate: The Beier CA16-IgM Test Kit demonstrates a high detection rate and good concordance. Data source: National Institute for Viral Disease Control and Prevention, Chinese CDC.
EV71-IgM Test Kit (Colloidal Gold) Performance Analysis
|
Sample |
No. of Cases |
EV71-IgM Positive |
EV71-IgM Negative |
Sensitivity |
Specificity |
|
EV71-IgM Positive Samples |
90 |
88 |
2 |
97.8% |
—– |
|
PCR Positive Samples / Non-HFMD Cases |
217 |
7 |
210 |
—– |
96.8% |
Results indicate: The Beier EV71-IgM Test Kit (Colloidal Gold) demonstrates high sensitivity and good specificity for testing serum from EV71-infected individuals. Data source: National Institute for Viral Disease Control and Prevention, Chinese CDC.
CA16-IgM Test Kit (Colloidal Gold) Performance Analysis
|
Sample |
No. of Cases |
CA16-IgM Positive |
CA16-IgM Negative |
Sensitivity |
Specificity |
|
CA16-IgM Positive Samples |
248 |
243 |
5 |
98.0% |
—– |
|
PCR Positive Samples / Non-HFMD Cases |
325 |
11 |
314 |
—– |
96.6% |
Results indicate: The Beier CA16-IgM Test Kit (Colloidal Gold) demonstrates high sensitivity and good specificity for detecting serum from CA16-infected individuals. Data source: National Institute for Viral Disease Control and Prevention, Chinese CDC.
Post time: Oct-30-2025