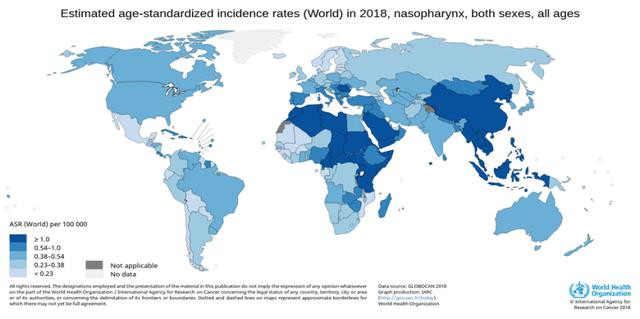
Nasopharyngeal (nay-zoh-fuh-RIN-jee-ul) carcinoma is cancer that occurs in the nasopharynx, which is located behind your nose and above the back of your throat.
Nasopharyngeal carcinoma is rare in the United States. It occurs much more frequently in other parts of the world — specifically Southeast Asia.
Nasopharyngeal carcinoma is difficult to detect early. That's probably because the nasopharynx isn't easy to examine and symptoms of nasopharyngeal carcinoma mimic those of other, more-common conditions.
Nasopharyngeal carcinoma mostly occurs in middle-aged and elderly people over 40 years old, and has obvious regional and familial characteristics, and the incidence rate in Guangdong ranks first in China, also known as "Guangdong cancer".
1.The Guidelines for the Diagnosis and Treatment of Nasopharyngeal carcinoma
In the 2021 Guidelines for the Diagnosis and Treatment of Nasopharyngeal Carcinoma, the Chinese Society of Clinical Oncology(CSCO) included serological detection methods in the Class I evidence for the diagnosis of nasopharyngeal carcinoma, and pointed out that the combination of EB-VCA-IgA and EB-NA1-IgA EB-virus antibodies can increase the early diagnosis rate of nasopharyngeal carcinoma by 3 times (21%~79%) and reduce the risk of death by 88%! The 2019 Expert Consensus on the Clinical Application of Markers for Nasopharyngeal Carcinoma also pointed out that EBV-EA-IgA is a marker of recent EBV infection or active proliferation of EBV, with a high degree of specificity, and is often used for nasopharyngeal cancer screening and early diagnosis.
The study shows that the three combined detections of EBV-VCA-IGA, EBV-EA-IGA and EB-NA1-IgA fully cover EBV gene spectrum, which effectively improves the sensitivity and specificity of nasopharyngeal carcinoma detection, minimizes missed detection, ensures the accuracy of disease prediction, and predicts the occurrence of disease 5-10 years in advance, which is suitable for large-scale nasopharyngeal cancer screening.
2.The VCA-IgA+EA-IgA+NA1-IgA produced by Beijing Beier can provide the early diagnosis protocol for Nasopharyngeal Carcinoma.
Magnetism particulate immuno chemistry luminescence method
| Product Name | Abbreviation |
| EB virus VCA-IgA antibody detection kit | EB-VCA-IgA |
| EB virus EA-IgA antibody detection kit | EB-EA-IgA |
| EB virus NA1-IgA antibody detection kit | EB-NA1-IgA |
Elisa Method:
| Product Name | Abbreviation |
| EB virus VCA-IgA Elisa kit | EB-VCA-IgA |
| EB virus EA-IgA Elisa kit | EB-EA-IgA |
| EB virus NA1-IgA Elisa kit | EB-NA1-IgA |
3.Product Performance
The VCA-IgA test kit produced by Beijing Beier can replace the EU standard kit for early detection and screening of nasopharyngeal carcinoma.
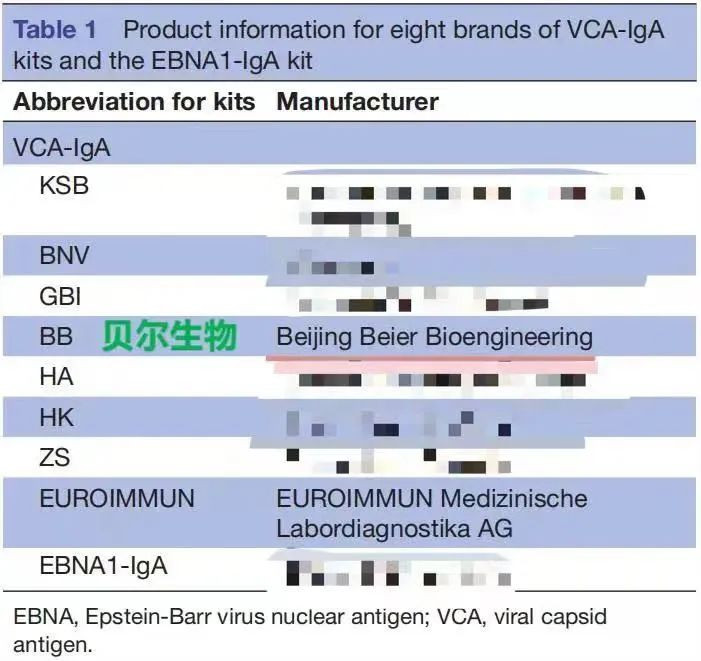
The British Medical Journal (BMJ) (impact factor 16.378) is One of the world's four leading medical journals. In 2017, a research team published a paper in the British Medical Journal (BMJ) "Evaluation of seven recombinant VCA-IgA ELISA kits for the diagnosis of nasopharyngeal carcinoma in China: a case-control trial".
In this paper, 200 patients with nasopharyngeal carcinoma (NPC) and 200 normal human serum samples (SYSUCC) from Sun Yat-sen University Cancer Center were studied and tested, and the performance of EB-VCA-IgA (ELISA) kits produced by 8 brand manufacturers in the domestic market was compared for performance evaluation. The conclusion is that the EBV-VCA-IgA (ELISA) kit produced by Beijing Beier has the same diagnostic effect as the EBV-VCA-IgA (ELISA) produced by the imported reagent Oumeng, and the EBV-VCA-IgA (ELISA) kit produced by Beijing Beier can replace the imported kit for the early detection and screening of nasopharyngeal carcinoma. The information of the brand manufacturers participating in the test is shown in Table 1, the test results are shown in Table 2, and the test conclusions are shown in Table 3.
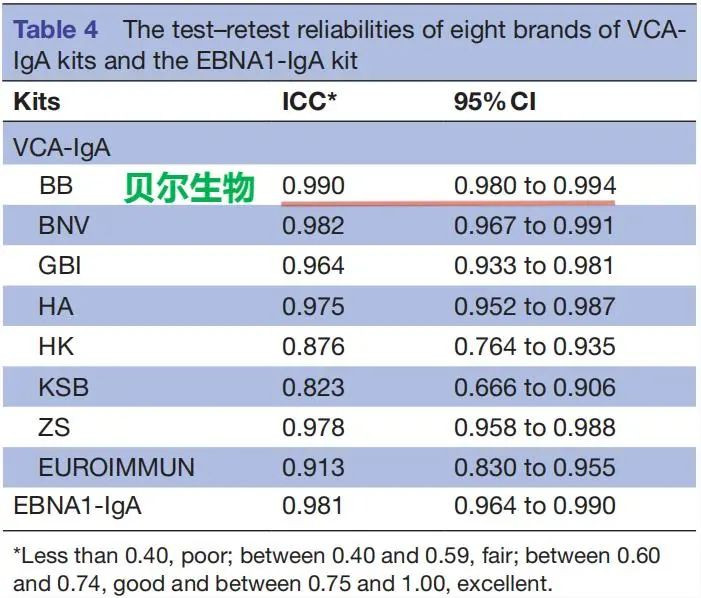
Test Conclusion
Three recombinant VCA-IgA kits-BB,HA and KSB- had diagnostic effects equal to those of the standard kit.They can be substituted for the standard kit and their combinations could be used in the early detection of and screening for NPC.
Post time: Feb-23-2023
